Our goal is to make organic synthesis faster, easier and more efficient. We focus on exploring catalysis to develop new ways to think about molecular assembly. In addition to discovering new reactions, we explore the mechanisms of these new processes to provide insight for further developments and aim our studies towards molecular structures that we think can be useful. We are particularly interested in complexity-building transformations, where multiple bonds are formed in a single process, and the ability to control reaction pathway and access divergent outcomes.
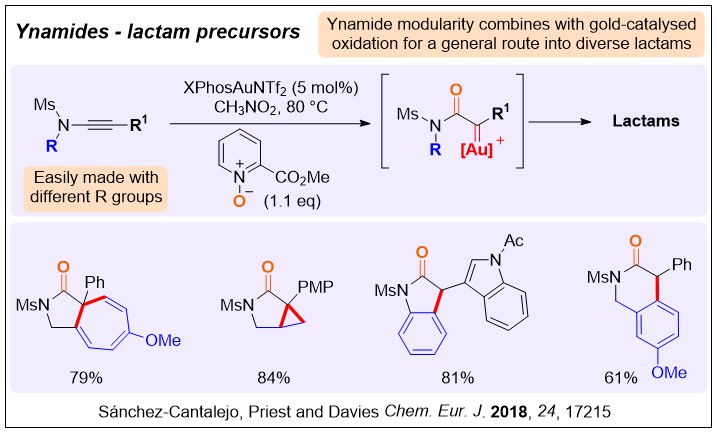
We have a major focus on gold catalysis due to its potential for tuneable and efficient synthesis, but are interested in all types of catalysis. The use of heteroatom-substituted alkynes in catalysis, such as ynamides and alkynyl thioethers, has been a major focus spanning almost all the different types of catalysis we have explored. Some examples are below and in the links and publication list.
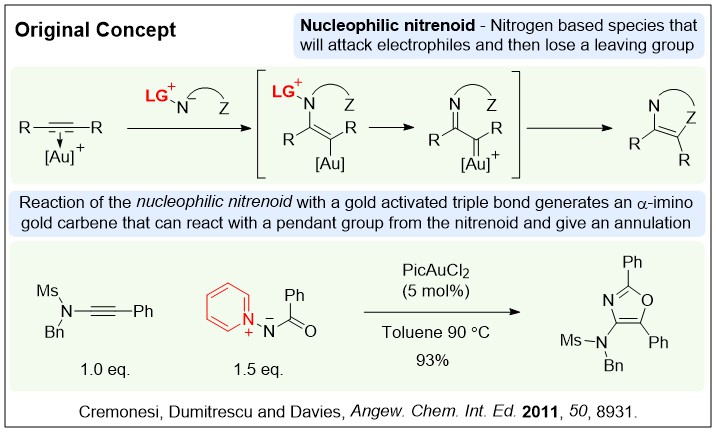
Nitrenoid chemistry
in 2011 we introduced a new annulation strategy for the synthesis of nitrogen heterocycles. This was based on using bench-stable nucleophilic nitrenoid reagents in combination with an alkyne and a catalyst to access and then quench alpha-imino gold carbene like reactivity patterns. We have been exploring this concept as a tool to allow rapid access into heavily substituted heterocycles.
Cycloisomerisations
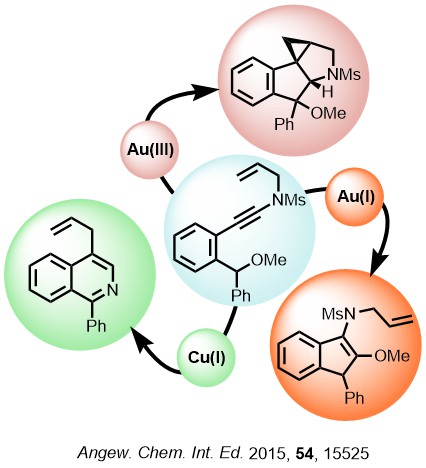
Cycloisomerisations represent an incredibly powerful tool for the formation of complex cyclic systems with inherent atom efficiency. Often different pathways are accessible and we explore how we can control outcomes by catalyst choice. We are particularly interested in using cycloisomerisation to test a reactivity concept whilst simultaneously accessing structurally useful motifs with potential for application. Our cycloisomerisation reactions have been triggered by processes including internal O and H atom transfer.
Gold carbenes
We are exploring different ways to access gold carbene reactivity patterns from alkynes. This allows us to tap into powerful reactivities from simple and readily accessible precursors. This approach avoids the synthetic and safety challenges associated with classical carbene precursors like diazo compounds, and has enabled a wide range of new transformations.
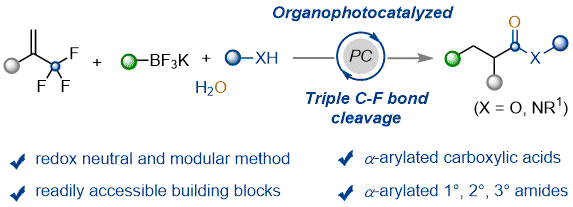
Photoredox catalysis
We have been exploring the use of both transition metal catalysed and organocatalysed photocatalysis as a tool to access new synthetic strategies, from ynamide polycyclisation in collaboration with the Shu group at SUSTech.