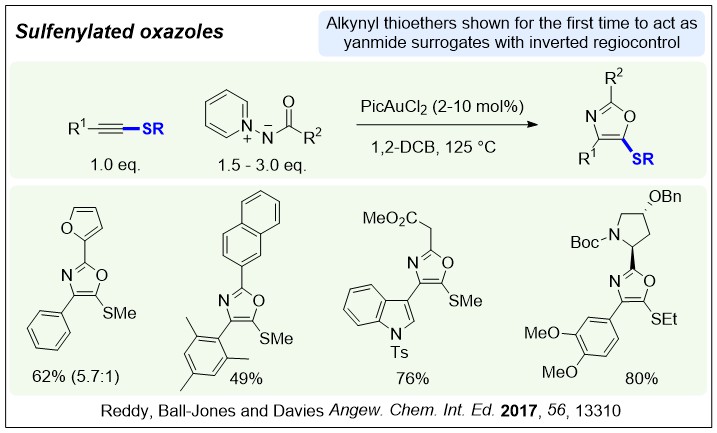
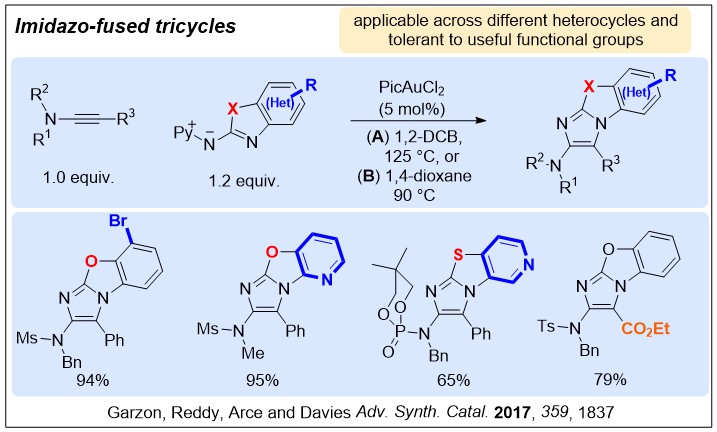
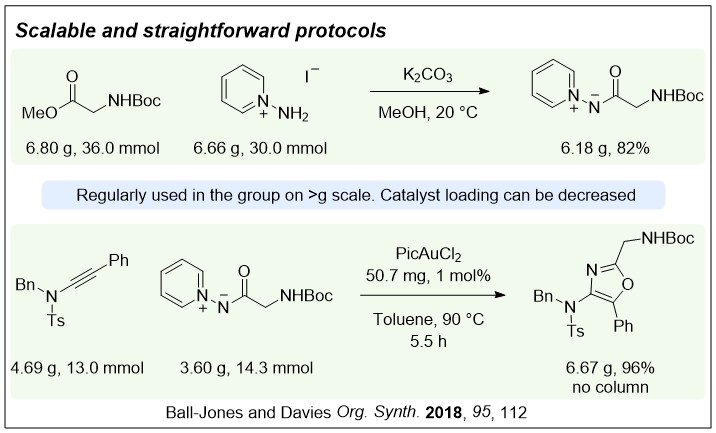
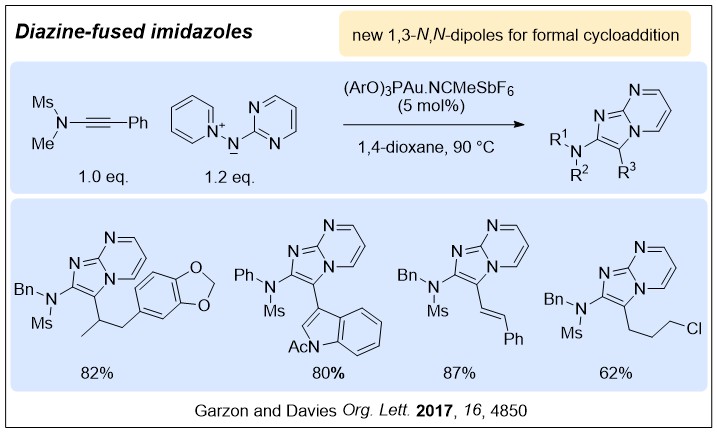
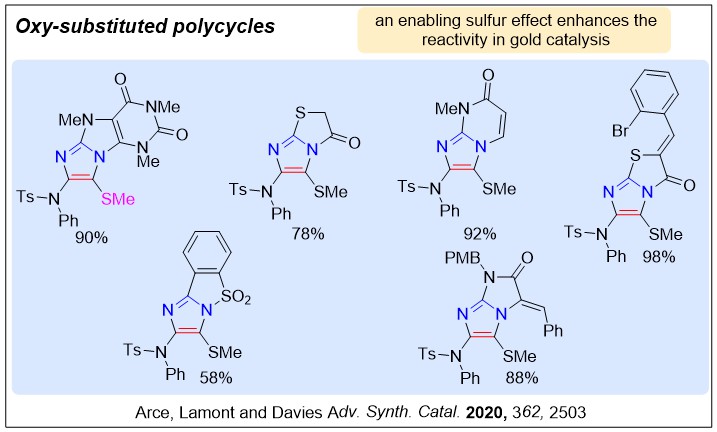
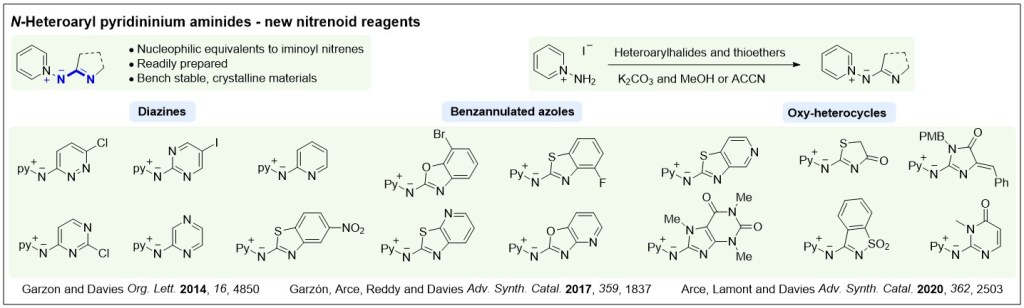
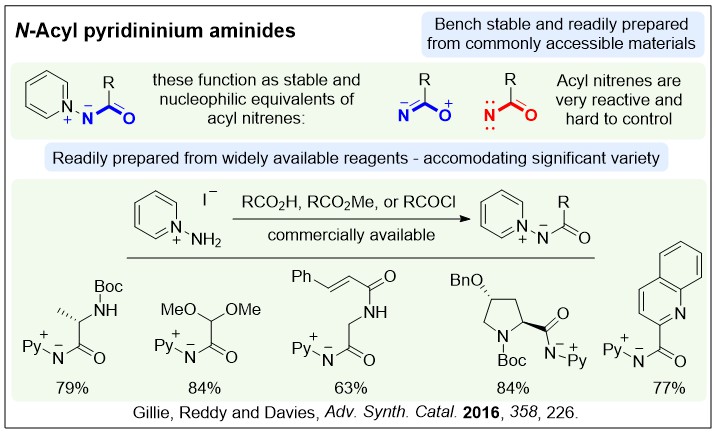
Nucleophilic nitrenoids are N-centred reagents that donate electron density to an electrophile, and then subsequently lose a leaving group, behaving as nitrene equivalents over a reaction. We have been exploring their use in gold-catalysed reactions to develop efficient new methods for nitrogen heterocycle synthesis.
We proposed that a nucleophilic nitrenoid could be used to deliver a new entry into cycloadditions, by reacting to generate a metal carbene from a pi-acid activated alkyne and delivering the means to quench it. In 2011 we reported the first example of this type of process. Since then, related approaches have been used in a wider variety of transformations.
For an early review of this area, see our invited review. A more recent summary of our work is here.
We are currently looking at expanding the potential of this approach and applying these methods to target synthesis.