We have pioneered the use of heteroatom (N, O, S)-substituted alkynes like ynamides and alkynyl thioethers as enabling tools for reaction discovery in gold catalysis.
Ynamides
These electron-rich alkynes are superb substrates for in gold catalysis, reacting via gold keteniminium species. These reactive electrophiles can be combined with a variety of nucleophiles to achieve regiospecific addition across the triple bond. We have been particularly interested in their use as precursors to metal carbene reactivity patterns in both intermolecular and intramolecular processes. Some examples are below:
Enabling novel annulation processes:
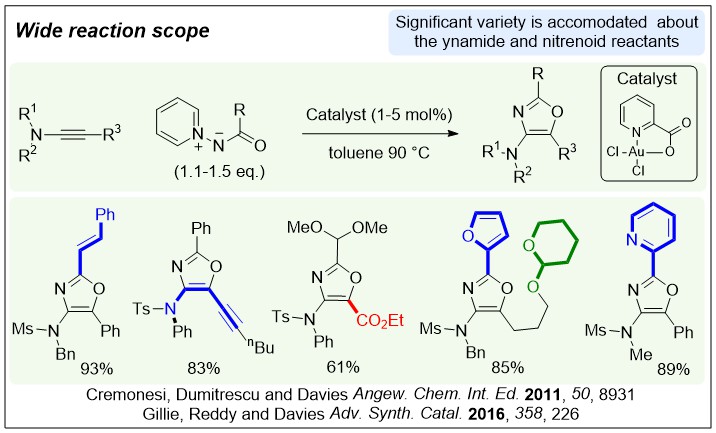
Intermolecular and selective synthesis of 2,4,5-trisubstituted oxazoles by a gold-catalysed formal [3+2] cycloaddition; P. W. Davies,* A. Cremonesi, L. Dumitrescu, Angew. Chem. Int. Ed. 2011, 38, 8931-8935.
As amino metal carbene precursors: We introduced the combination of a nucleophilic oxidant with an ynamide to access alpha-amido gold carbene reactivity patterns. This approach has been used by us and many other groups to generate a wider range of transformations.
Site-specific introduction of gold-carbenoids by intermolecular oxidation of ynamides or ynol ethers; P. W. Davies,* A. Cremonesi, N. Martin, Chem. Commun. 2011, 47, 379-381.
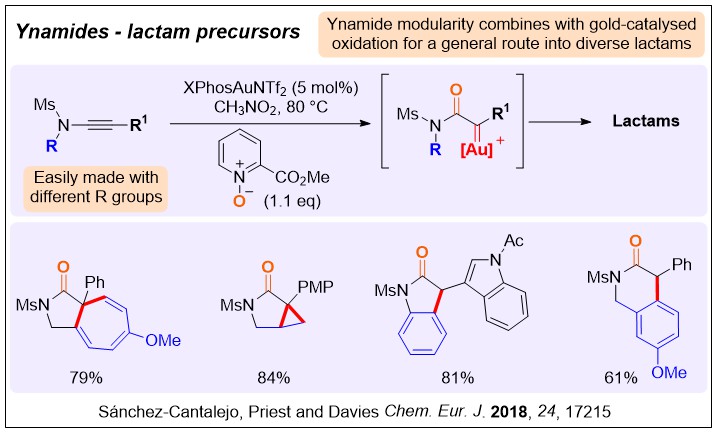
A Gold Carbene Manifold to Prepare Fused γ‐Lactams by Oxidative Cyclisation of Ynamides, F. Sanchez-Cantalejo, J. D. Priest, P. W. Davies,* Chem. Eur. J. 2018, 24, 17215-17219.
Diverting reaction pathways: We have shown that an ynamide can be used to override geometric bias in intramolecular reactions, providing access into previously unexplored carbene environments and enabling novel transformations.
The first example of a 1,2-nitrogen migration onto a gold carbene:

1,2-N-Migration in a Gold-Catalysed Synthesis of Functionalised Indenes by the 1,1-Carboalkoxylation of Ynamides; H. V. Adcock, T. Langer, P. W. Davies,* Chem. Eur. J. 2014, 20, 7262-7266.
Generating sp3-sp3 systems from triple bonds by formal CH insertion of an ynamide:
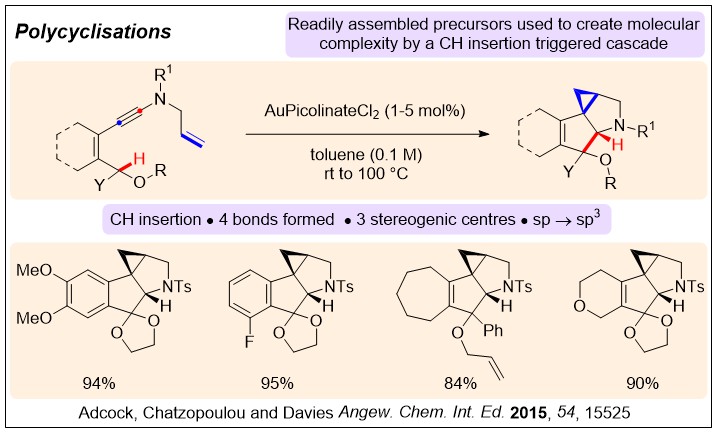
Divergent C-H Insertion-Cyclization Cascades of N-Allyl Ynamides; H. V. Adcock, E. Chatzopoulou, P. W. Davies* Angew. Chem. Int. Ed. 2015, 54, 15525-15529.

Gold-catalysed cycloisomerisation of ynamides to access 2,2-disubstituted tetrahydrothiophene motifs, P. Heer Kaur and P. W. Davies* Synlett, 2021, 32, 897-900.
Alkynyl thioethers
A growing area of interest in the group concerns the use of sulfur substitution in alkynes. We showed that these species, far from poisoning gold catalysts actually enhance reactivity in some gold catalysed processes and that there is also an intriguing regiocontrolling processes. We are exploring the underlying aspects of this reactivity whilst also exploring the potential to generate efficient new synthetic methods. See:
Alkynyl thioethers in gold-catalysed annulations to form oxazoles, R. J. Reddy, M. P. Ball-Jones, P. W. Davies,* Angew. Chem. Int. Ed. 2017, 56, 13310-13313.

Regiodivergent Synthesis of 4- and 5-Sulfenyl Oxazoles from Alkynyl Thioethers Prakash Sekar, Aniket Gupta, Laura E. English, Clare E. Rabbitt, Louise Male, Andrew R. Jupp* and Paul W. Davies* Chem. Eur. J. 2024 in press

Gold(I)-Catalyzed Synthesis of 3-Sulfenyl Pyrroles and Indoles by a Regioselective Annulation of Alkynyl Thioethers, P. E. Simm, P. Sekar, J. Richardson, P. W. Davies* ACS Catalysis, 2021, 6357–6362.
Sulfenyl Ynamides in Gold Catalysis: Synthesis of Oxo-functionalised 4-Aminoimidazolyl Fused Compounds by Intermolecular Annulation Reactions, E. M. Arce, S. G. Lamont, P. W. Davies,* Adv. Synth. Catal. 2020, 362, 2503-2509.
Alkynyl sulfoxides
We have shown that an alkynyl sulfoxides can be used to access desirable carbene substitution patterns that had proven previously elusive, and used this to access novel bridged bicyclic motifs.
Alkynyl sulfoxides as α-sulfinyl carbene equivalents: Gold-catalysed oxidative cyclopropanation, M. J. Barrett, G. F. Khan, P. W. Davies,* R. S. Grainger* Chem. Commun. 2017, 53, 5733-5736.